Biosimilar medical drugs
A biosimilar drug is a biological medicinal product similar to another biological product, the „original product,” which has already been approved in the European Union. Over fifteen years of experience show that biosimilar drug quality, effectiveness, and safety are identical to the original product. However, the price is different. Biosimilars are at least 30 percent cheaper than the original.
A biological medicinal product is made out of biological resources such as living cells or organisms. The production tends to be more complicated than it is in the case of chemical substances. Most are produced using biotechnologies, often with the use of complex cell systems and recombinant DNA technology.
A biosimilar preparation is a biological medicinal product resembling a different biological product which has been approved within the European Union (it is a reference product – an original biological product.) A biosimilar product is not considered to be a generic of a biological drug. This is mostly because the natural variability and more complex production of biological drugs do not allow an exact replication.
Safe and effective
The registration of biosimilar products takes place on a pan-European level. It is conditioned by a rigorous testing of chemical and physical properties, which is completed by preclinical and clinical studies. Therefore, the registration is conditioned by demanding tests that prove that the biosimilar product is as effective and safe as its original predecessor (reference product) and that its active ingredient behaves the same way in the patient’s body (bioequivalence).
The European Medicines Agency (EMA) approved the first biosimilar product in 2006. The evidence gained during the 15 years of clinical experience shows that biosimilar products approved by the EMA can be used as safely and effectively in all approved indications as other biological products. The list of all EU approved biosimilar drugs is available on the EMA webpage.
In September 2022, EMA and the Heads of Medicines Agencies (HMA) issued a joint statement confirming that biosimilar medicines approved in the European Union (EU) are interchangeable with their reference medicine or Heads of Medicines Agencies (HMA) issued a joint statement confirming that biosimilar medicines approved in the European Union (EU) are interchangeable with their reference medicine or with an equivalent biosimilar. More info you can find here.
The share of biological preparations in cancer treatment keeps growing.
The biological preparation rituximab, the first monoclonal antibody approved in the EU for cancer indication, presented an important improvement of the prognosis of leukaemia and is now considered a part of „standard care“. However, there are already other biosimilar preparations containing medicinal substances for the treatment of cancer such as trastuzumab and bevacizumab on the market, which contributes to the availability of treatment.
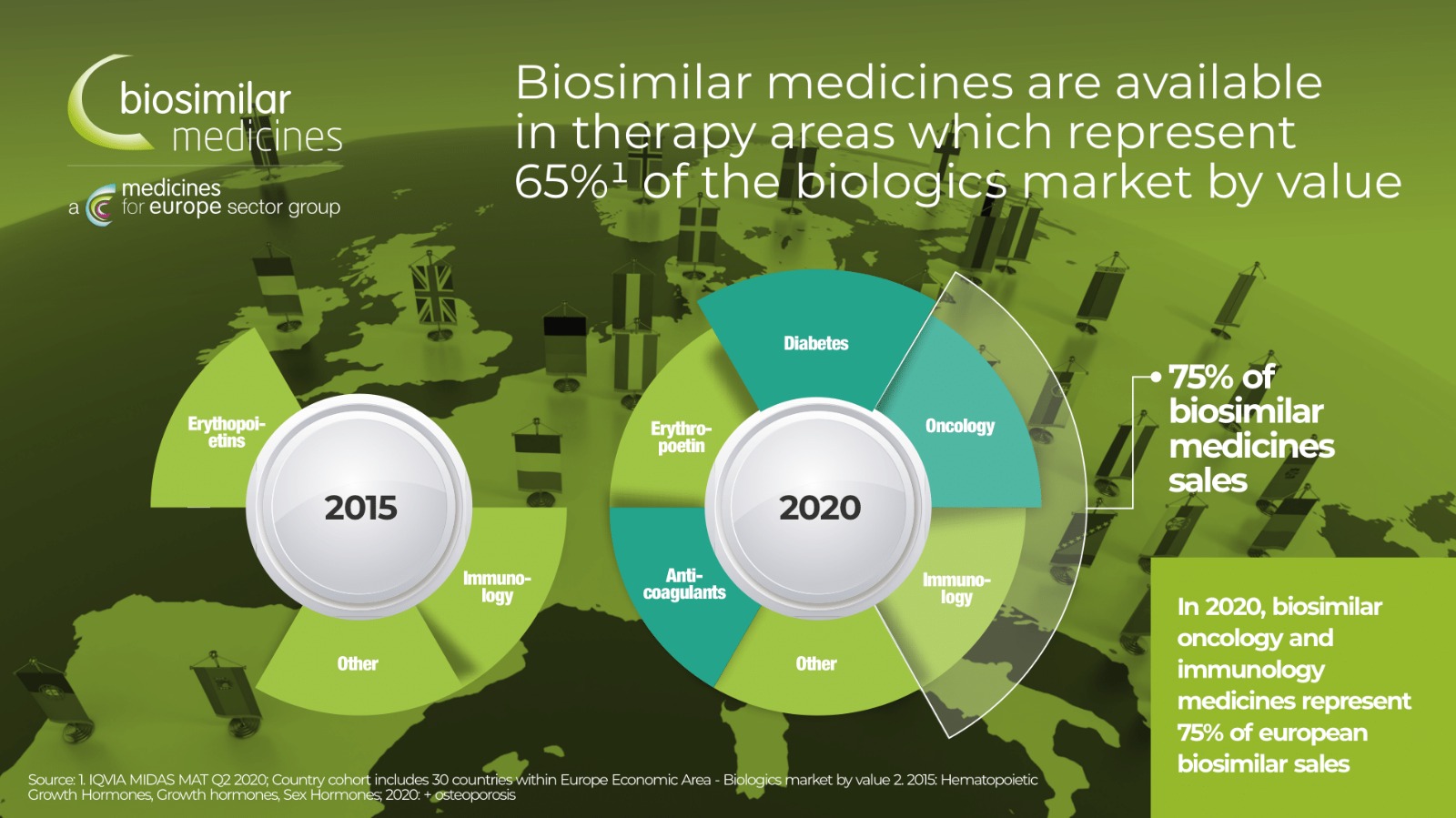
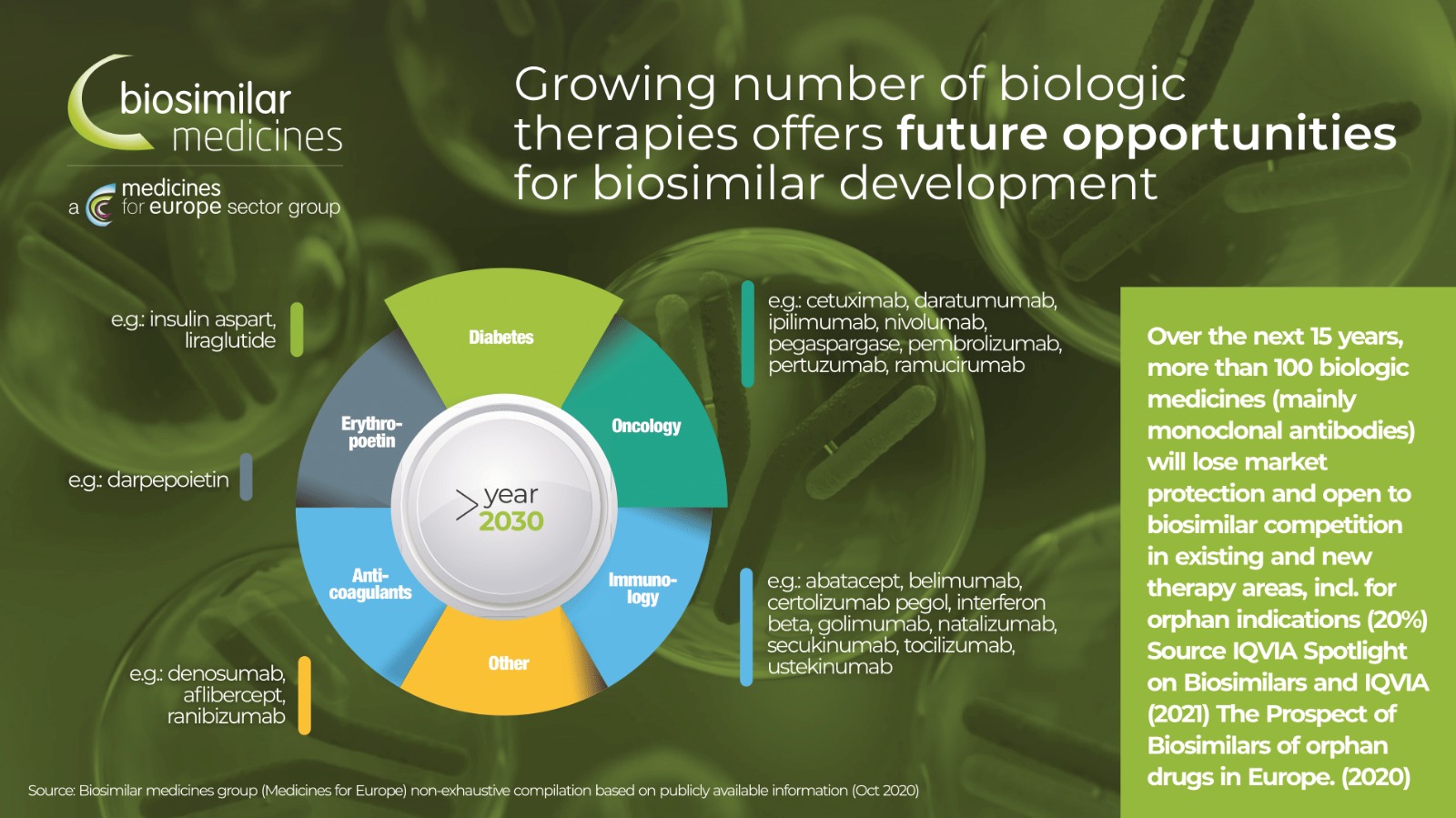
Cancer treatment expenses continue to grow as new, more targeted therapies enter the market, putting pressure on health budgets and, in some countries, significantly affecting the patient's access to treatment. Over the next 10 years, many biological products will lose their patent protection, opening space for biosimilar products in oncology.
Care accessibility
With the entrance of biosimilar medical drugs onto the market, just as in the case of the entry of generics, the price of medicinal products, intended for a treatment of a given disease, reduces due to the competition of more products. As a result, more patients can be treated while maintaining the same level of total expenses. How many patients will be treated for the same cost is also dependent on the price reduction of medicinal products and the ratio of medicinal product use – what is the presumed penetration rate of the biosimilar medical drug in the given group of products.
The price of medicinal products is reduced both in connection with the rules established by the Act of Public Health Insurance and the competition of medicinal products in the given group.
The penetration rate of biosimilar medical drugs is affected by various factors – the specific experience of doctors, the way of awarding public contracts in which the medical drugs are purchased for hospitals, the existence of significant comparison studies and specialised literature of the regulatory mechanisms, e. g. changing the dispensing method with the possibility of dispensing the prescription-only medicinal product.
What exactly does the entrance of a biosimilar drug onto the market cause? Below we present the consequences of high penetration rate of biosimilar drugs and a lower penetration in a large group of medicinal products.
Medicinal products containing a medicinal substance pegfilgrastim
These medicinal products are used with oncology and haemato-oncology patients for the stimulation of haematopoiesis after its suppression, for example, during cytostatic treatment or radiation.
At the moment, there are five registered and marketed medicinal products containing this medicinal substance. In addition to the original biological product Neulasta, there have been other biosimilar medicinal drugs that have entered the public health system since 2018.
After three years of entry, biosimilar medicinal products represent 94 percent of the consumption of medicinal products containing the above-mentioned medicinal substance. At the same time, the pricing and reimbursement regulation and competition between the medicinal products have led to a price reduction of 45 percent. In the monitored period, there was also a slight increase in expenses of medicinal products by 17 percent, which represents 14 million Czech crowns. However, during the overall expense increase, there was also an increase of treated patients, namely by 114 percent, which means that twice as many patients can be treated for the same cost.
Medicinal products containing a medicinal substance adalimumab
Medicinal products containing adalimumab are used with moderate and severe cases of autoimmune diseases such as Crohn’s disease, ulcerative colitis, psoriasis, rheumatoid arthritis, and ankylosing spondylitis (Bechterev’s disease). This is a large therapeutic group which represents costs of over one billion crowns per year. At the moment, there are five registered and marketed products containing this medicinal substance.
Compared to medicinal products containing pegfilgrastim, there is a much lower representation of biosimilar medicinal products. After three years, they presented only 31 percent of the total medicinal products used containing this medicinal substance.
Three years after the entry of biosimilar drugs, the total expenses have dropped by 242 million Czech crowns (19 percent), and there has been an average 42 percent decrease in the price of an individual package. In these conditions, the number of healable patients has grown by 40 percent.
From the given examples, it is clear that the entry of biosimilar drugs has a positive effect on both the expenses of the public health insurance and the accessibility of treatment by the given medications for Czech patients.
These are the reasons that ČAFF tries to present these results to those who can ensure a more extensive use of the potential of biosimilar drugsin the Czech Republic.